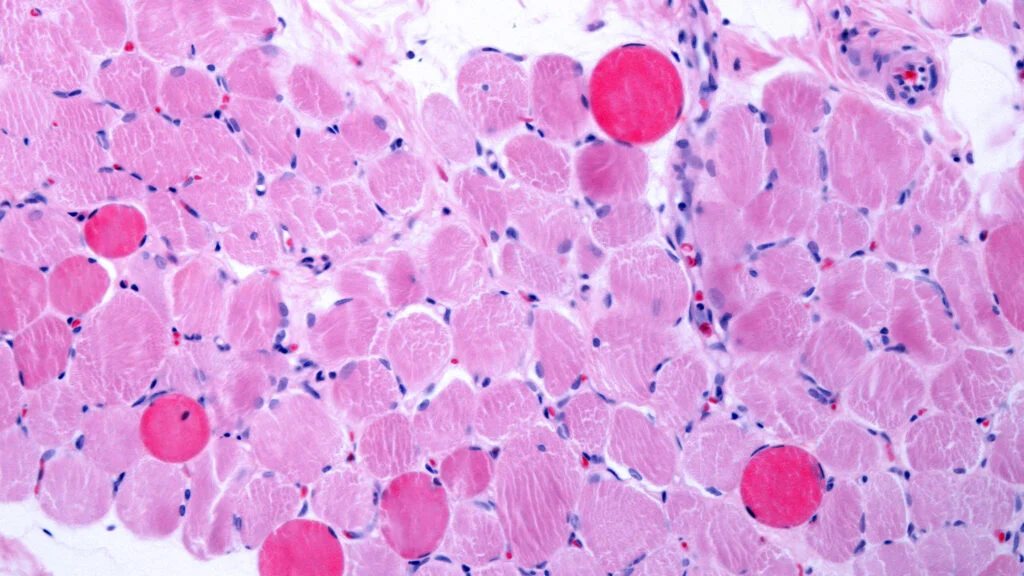
Stat Capricor Says Its Cell Therapy Improves Heart and Muscle Function in Duchenne Patients
Capricor Therapeutics announced on Wednesday that its cell therapy has significantly improved both muscle and heart function in patients suffering from Duchenne muscular dystrophy. These dual improvements met the primary objectives of a Phase 3 clinical trial. The positive outcomes are expected to strengthen the future prospects of the treatment, especially amid an ongoing and contentious regulatory review process.
In July, the Food and Drug Administration (FDA) rejected Capricor’s treatment, known as deramiocel. The FDA stated that the application, which was based on mixed results from an earlier study, did not provide “substantial evidence of effectiveness.” This decision reportedly came from Vinay Prasad, the FDA’s top regulator for cell and gene therapies. He overruled FDA staff members who had supported the approval of the therapy.
Positive Phase 3 Results Could Influence Regulatory Decisions
Capricor’s CEO, Linda Marbán, expressed confidence that the new, positive data from the larger, placebo-controlled Phase 3 study will be persuasive enough to reverse the FDA’s earlier rejection. The recent trial demonstrated significant improvements in both muscle strength and heart function, which are critical factors in treating Duchenne muscular dystrophy. These results address the FDA’s previous concerns about the therapy’s effectiveness.
The Phase 3 study’s success marks an important milestone for Capricor. It provides stronger evidence supporting deramiocel’s potential benefits for patients with this serious muscle-wasting disease. The company hopes that these findings will lead to a favorable reconsideration of the treatment by regulatory authorities.
Stat Capricor Says Its Therapy Could Change the Outlook for Duchenne Muscular Dystrophy
The announcement that stat Capricor says its cell therapy improves heart and muscle function in Duchenne patients highlights a significant advancement in the search for effective treatments. Duchenne muscular dystrophy is a debilitating condition that affects both skeletal muscles and cardiac function. By targeting both aspects, deramiocel offers a comprehensive approach to managing the disease.
Capricor’s latest data reinforce the therapy’s ability to deliver meaningful clinical benefits. The company’s leadership believes this will be a key factor in overcoming previous regulatory hurdles. As the FDA continues its review, the new evidence may help tip the balance in favor of approval.
In summary, Capricor Therapeutics has reported that its cell therapy significantly enhances muscle and heart function in Duchenne muscular dystrophy patients. These results meet the main goals of a Phase 3 trial and could influence the FDA’s regulatory stance. CEO Linda Marbán remains optimistic that the new findings will lead to a reversal of the earlier rejection, potentially offering a new treatment option for those affected by this challenging disease.
For more stories on this topic, visit our category page.
Source: original article.